Pd-Catalyzed disilylation: an efficient route to 2,2′-bis(trimethylsilyl)biphenyls via trapping transient dibenzopalladacyclopentadienes with hexamethyldisilane†
Abstract
A palladium-catalyzed disilylation reaction of 2-iodobiphenyls with hexamethyldisilane via trapping transient dibenzopalladacyclopentadienes has been achieved. Diversely functionalized 2,2′-bis(trimethylsilyl)biphenyls have been synthesized in good to excellent yields under mild conditions. Moreover, 2,2′-bis(trimethylsilyl)biphenyls readily transformed into 2,2′-dihalobiphenyls, which could be used to synthesize carbazole, dibenzo[b,d]thiophene and dibenzo[b,d]selenophene polycyclic heteroarenes.
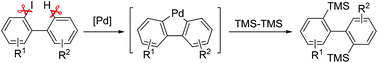


 Please wait while we load your content...
Please wait while we load your content...