Ion-pair electrostatic attraction-enhanced donor–acceptor interactions between the prototypic 1,4-dialkoxybenzene-viologen binding mode in water†
Abstract
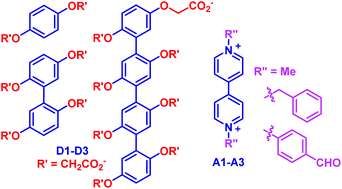
Three electron-rich donor molecules D1–D3, which consist of one, two or four 1,4-bis(carboxylatomethoxy)benzene units, respectively, have been prepared. Donor–acceptor interactions between the di-, tetra- and octaanionic donors and electron-deficient bipyridinium derivatives A1–A3, which bear two methyl, benzyl, or 4-formylphenyl subunits, respectively, have been investigated using X-ray diffraction analysis (for the complex of D2 and A1) and 1H NMR and absorption experiments. It was revealed that D1 and D2 formed 1 : 1 donor–acceptor complexes with the three acceptors, while D3 formed 1 : 2 complexes with them. All the complexes exhibited high water-solubility. The crystal structure of complex D1·A1 showed that the biphenyl unit of D1 and the bipyridinium unit of A1 stacked alternately in the new perfect face-to-face pattern. By using isothermal titration calorimetric titration experiments, association constants for the 1 : 1 and 1 : 2 complexes in water were determined to be as high as 1.3 × 104 M−1 and 2.6 × 1011 M−2, respectively. The results supported that the intermolecular ion-pair electrostatic attraction between the carboxylate anions and the bipyridinium dication remarkably enhanced the intermolecular donor–acceptor interaction in water.


 Please wait while we load your content...
Please wait while we load your content...