Substrate selective synthesis of indole, tetrahydroquinoline and quinoline derivatives via intramolecular addition of hydrazones and imines†
Abstract
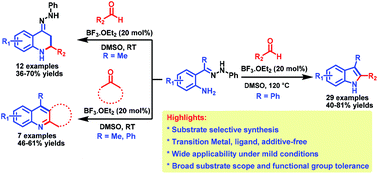
A transition-metal-free, substrate selective synthesis of 2,3-diaryl indoles has been developed via the intramolecular addition of hydrazones (bearing no α-H, derived from 2-aminobenzophenones and phenylhydrazines) to in situ generated imine intermediates. However, the hydrazones derived from 2-aminoacetophenones (bearing α-H) produced the corresponding 4-hydrazono-tetrahydroquinolines in a substrate selective manner under the optimized reaction conditions at room temperature. Moreover, differently substituted quinoline derivatives were obtained from the nucleophilic addition of in situ generated enamines derived from ketones and (E)-2-(aryl/methyl(2-phenylhydrazono)methyl)anilines.


 Please wait while we load your content...
Please wait while we load your content...