Synergistic catalysis: enantioselective cyclopropanation of alkylidene benzoxazoles by Pd(ii) and secondary amine catalysis. Scope, limitations and mechanistic insight†
Abstract
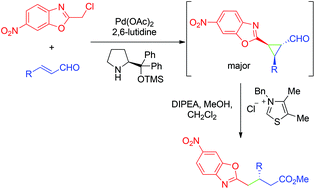
The reaction of alkyl-benzoxazoles with enals has been reported using a synergistic approach. It consists of the activation of enals with a secondary amine catalyst and the activation of benzoxazoles with a metal Lewis acid. This approach has also been applied to the synthesis of cyclopropanes with excellent results. We demonstrated the applicability of this reaction in a cascade reaction (cyclopropanation + ring opening) that circumvents some of the limitations of the simple Michael addition between alkyl-benzoxazoles and enals. Finally, mechanistic studies have been reported to explain the stereoselectivity of the cyclopropanation reaction that renders an unusual cis conformation.


 Please wait while we load your content...
Please wait while we load your content...