The mechanism of copper-catalyzed oxytrifluoromethylation of allylamines with CO2: a computational study†
Abstract
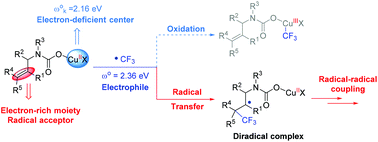
The trifluoromethyl group (CF3) is a very important functional group. The mechanism of transition metal catalyzed trifluoromethylation has received considerable attention in recent years. In this work, the detailed mechanism of the copper-catalyzed oxytrifluoromethylation of allylamines with CO2 was investigated by density functional theory (DFT) calculations. Differing from the previous Cu(I)–Cu(III) catalytic cycle, the results show that the reaction proceeds through a Cu(I)–Cu(II) catalytic cycle. Deprotonation of allylamines initially occurs with the assistance of a copper catalyst followed by CO2 insertion. In the presence of Togni reagent II, the copper(I) carboxylate species can then be oxidized to the copper(II) dicarboxylate intermediate along with the formation of a free trifluoromethyl radical, which then attacks the alkene moiety to generate the electrophilic addition diradical adduct. The spiro ring is constructed by a carboxylate-delivered radical–radical cross-coupling procedure. In addition, the calculated global electrophilicity shows that the copper(III) intermediate cannot be generated from the combination of the electron deficient copper center and the electrophilic trifluoromethyl radical. Frontier molecular orbital analysis indicates that the Togni reagent II is activated by neutral Cu(I) rather than the cationic Cu(I) species. The origin of the diastereoselectivity can be mainly attributed to the repulsion between the trifluoromethyl group and the carbonyl moiety.


 Please wait while we load your content...
Please wait while we load your content...