Synthesis of N-sulfenyl-sulfoximines and -sulfenamides through a metal-free N–H/S–H dehydrocoupling reaction†
Abstract
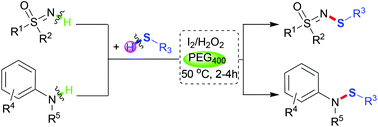
A metal-free, iodine-catalyzed N–H/S–H dehydrocoupling reaction of sulfoximines and anilines with various thiols to construct sulfur–nitrogen bonds is herein presented. A variety of structurally diverse N-sulfenylsulfoximines and sulfenamides were prepared with up to 97% yield. The reaction was carried out in the presence of hydrogen peroxide in PEG400 under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...