Ultra-fast Suzuki and Heck reactions for the synthesis of styrenes and stilbenes using arenediazonium salts as super-electrophiles†
Abstract
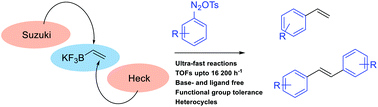
The super-electrophilic properties of arenediazonium salts have been exploited for achieving ultra-fast palladium-catalyzed coupling reactions with turnover frequencies up to 16 200 h−1. These ultra-fast coupling reactions have been exemplified with the synthesis of prone-to-polymerization styrenes within seconds through Suzuki cross-couplings with potassium vinyltrifluoroborate. Heterocycles and functional groups such as halides were well tolerated. The ambivalent properties of potassium vinyltrifluoroborate also allowed the development of ultra-fast sequential Suzuki–Heck reactions for the preparation of symmetrical and unsymmetrical stilbenes within minutes.


 Please wait while we load your content...
Please wait while we load your content...