Synthesis of substituted 4-hydroxyalkyl-quinoline derivatives by a three-component reaction using CuCl/AuCl as sequential catalysts†
Abstract
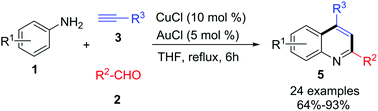
A new method to construct 4-hydroxyalkyl-quinoline derivatives is described via Cu(I) and Au(I) sequential catalyzed cyclization of anilines with aldehyde derivatives and aliphatic alkynes, respectively. The three-component cascade reaction provides an efficient approach for easy access to various new quinoline-based derivatives and quinoline containing an aliphatic chain substituent at the 4-position with high yields.


 Please wait while we load your content...
Please wait while we load your content...