Aliphatic C–H azidation through a peroxydisulfate induced radical pathway†
Abstract
A persulfate induced radical process to transform aliphatic tertiary C–H bonds into organic azides under transition-metal-free conditions has been developed. This method exhibits good chemo- and regioselectivity. A wide range of functional groups, including esters, halogens, azides, and dipeptides, are all tolerated well through the radical pathway, thus expanding the potential application of this method.
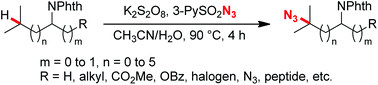

 Please wait while we load your content...
Please wait while we load your content...