An excellent cryogenic magnetic cooler: magnetic and magnetocaloric study of an inorganic frame material†
Abstract
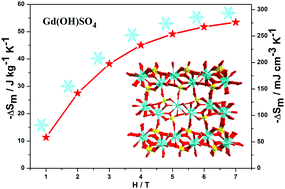
The cryogenic magnetic coolers based on the magnetocaloric effect (MCE) of paramagnetic compounds usually need relatively large fields to realise a practical cooling performance. Thus, it is of significance to search for molecule-based cryogenic magnetic coolers with high performance of the MCE under low fields (smaller than 2 T), considering the ready availability of low fields by commercial Nd–Fe–B permanent magnets. In this work, the crystal structure, magnetic susceptibility and isothermal magnetization for the inorganic compound Gd(OH)SO4 (1) have been investigated. The title compound exhibits a 3D structure with Gd-oxygen chains as the supramolecular building units. Magnetic characterisations indicated that adjacent GdIII ions show weak magnetic couplings in 1. Because of the integration of large metal/ligand mass ratio, weak magnetic couplings and a dense framework, the observed maximum entropy change (−ΔSmaxm) for 1 was up to 53.5 J kg−1 K−1 or 276 mJ cm−3 K−1 for ΔH = 7 T and T = 2 K, comparable to the performance of commercial gadolinium gallium garnet Gd3Ga5O12 (GGG, −ΔSmaxm = 38.4 J kg−1 K−1 or 272 mJ cm−3 K−1 with ΔH = 7 T). Notably, the −ΔSmaxm of 1 still reaches 27.5 J kg−1 K−1 at T = 2 K and ΔH = 2 T, and 38.3 J kg−1 K−1 at T = 2 K and ΔH = 3 T, which already surpasses GGG (about 24 J kg−1 K−1 with ΔH = 3 T) and makes 1 a superior cryogenic magnetic cooler for low-temperature applications, even in low fields.
- This article is part of the themed collection: Materials Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...