Ultrafine Rh nanoparticle decorated MoSe2 nanoflowers for efficient alkaline hydrogen evolution reaction†
Abstract
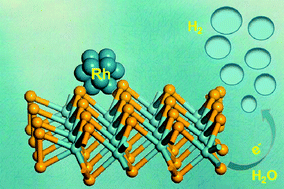
The development of highly efficient and stable electrocatalysts for the hydrogen evolution reaction (HER) under alkaline media is of great importance due to its sluggish water dissociation kinetics. In this work, ultrafine Rh nanoparticle decorated MoSe2 nanoflowers have been synthesized through a colloidal synthetic method. When further used as electrocatalysts for HER in alkaline media, the experimental results demonstrate that 8.2% Rh–MoSe2 nanoflowers exhibit excellent catalytic activity with a small overpotential of 73 mV to obtain the current density of 10 mA cm−2 with good stability, which is much higher than that of MoSe2 (264 mV) and even close to 20% commercial Pt/C (48 mV). The improved HER performance of Rh–MoSe2 nanoflowers may be ascribed to the synergistic catalytic effects between Rh and MoSe2 and strong electronic interactions among Rh, Mo and Se at the atomic level.


 Please wait while we load your content...
Please wait while we load your content...