Rapid fabrication of SnO2 nanoparticle photocatalyst: computational understanding and photocatalytic degradation of organic dye†
Abstract
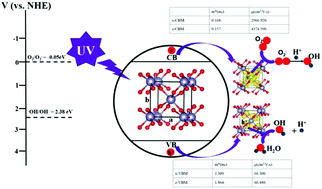
Metal oxides have attracted an increasing attention for the photo-degradation of organic containments. Deep understanding of the physical parameters correlated with the photocatalytic process is critical and beneficial for finding an efficient and robust photocatalyst. Herein, by taking SnO2 as a prototypical model, we systematically study exciton energy, effective mass, carrier mobility and partial charge density based on the density functional theory (DFT). We demonstrate that the obtained exciton energy is quite low and can be effectively dissociated into charge carriers at room temperature. The estimated carrier mobility of electrons is about 50 times greater than that of holes. More interestingly, analyzing partial charge density on the top of the valence band reveals that the photocatalytic oxidation reaction site would occur on the O-p state. Experimentally, SnO2 nanoparticles have been synthesized by a simple method and characterized by Powder XRD and TEM. With the photocatalyst SnO2, more than 90% methyl blue (MB) and Rhodamine B (RhB) are degraded under the UV light irradiation within 50 min and 270 min, respectively. Trapping experiments reveal that ˙OH are the main active species to oxidize the organic dye and mainly originate through the oxidation of holes (h+). This study establishes an in-depth understanding of electronic structure and photocatalysis and provides insights into the designing of new photocatalytic materials.


 Please wait while we load your content...
Please wait while we load your content...