Hydrothermal synthesis of Sn-Beta zeolites in F−-free medium†
Abstract
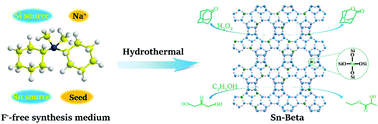
The Sn-Beta zeolite is a unique solid Lewis acid catalyst, showing great potential in the Baeyer–Villiger oxidation reaction as well as the biomass conversion reactions. The direct hydrothermal synthesis of Sn-Beta often involved harmful F− ions when using tetraethylammonium hydroxide (TEAOH) as the structure-directing agent (SDA). In this study, N-cyclohexyl-N,N-dimethylcyclohexanaminium hydroxide was used as the SDA to synthesize the Sn-Beta zeolite in F−-free medium. The combination of a small amount of Na+ ions and pure-silica Beta seeds proved to be critical for the successful synthesis of the highly crystalline Sn-Beta zeolite. The Si/Sn ratio could be tuned in the range of 60–200 by varying the gel Na/Si ratio. To obtain highly crystalline Sn-Beta zeolites, the removal of SDA molecules via calcination should be performed subsequent to NH4+ ion-exchange. With a smaller crystal size and stronger Lewis acidity, the obtained Sn-Beta showed a higher catalytic activity than that synthesized in F− medium with a comparable Sn content in both the Baeyer–Villiger oxidation reaction and isomerization–esterification reaction.
- This article is part of the themed collection: In honour of Professor Xu Ruren for his forty-year contribution in zeolitic materials research


 Please wait while we load your content...
Please wait while we load your content...