Magnetic mesoporous TiO2 microspheres for sustainable arsenate removal from acidic environments†
Abstract
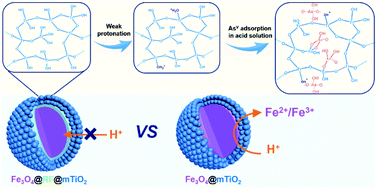
Carcinogenic arsenic pollution in ground water seriously threatens the health and lives of humans all over the world. It is highly desirable to fabricate new materials for sustainable arsenate removal with high capacities, stabilities and recyclabilities. In this study, we demonstrate that uniform magnetic core–shell structured Fe3O4@Resorcinol-Formaldehyde@mesoporous TiO2 microspheres (denoted Fe3O4@RF@mTiO2) can function as excellent adsorbents for the fast removal of arsenate (AsV) in acidic environments with very high efficiency. The mesoporous TiO2 outer shell (50 nm in thickness) endows them with a high surface area of 337 m2 g−1 and a large pore volume of 0.42 cm3 g−1, thus resulting in a fast adsorption rate (1.16 g mg−1 h−1) and a high adsorption capacity (up to 139 mg g−1) calculated using the Langmuir model at a pH of 3. The inner Fe3O4 core (130 nm in diameter) makes separation facile from wastewater using a magnet. Moreover, the hydrophobic properties of the RF interlayer (10 nm in thickness) are increased after calcination at 200 °C, and this can protect the inner Fe3O4 cores against etching from acid solutions over long cycles. In addition, the study of the AsV adsorption mechanism on the core–shell mesoporous Fe3O4@RF@mTiO2 microspheres shows the existence of electrostatic forces and surface complexation interactions between arsenate and partially crystallized TiO2. Benefiting from all of these advantages, the multilayer magnetic core–shell structured design is expected to be a promising nanomaterial for long-term wastewater treatment.
- This article is part of the themed collections: Inorganic Chemistry Frontiers HOT articles for 2018 and In honour of Professor Xu Ruren for his forty-year contribution in zeolitic materials research


 Please wait while we load your content...
Please wait while we load your content...