Self-assembly of tetra-nuclear lanthanide clusters via atmospheric CO2 fixation: interesting solvent-induced structures and magnetic relaxation conversions†
Abstract
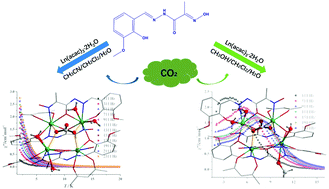
Two systems of carbonate complexes were constructed by employing a newly prepared Schiff based ligand (H2L; H2L = 2-(hydroxyimino)-2-[(3-methoxyl-2-hydroxyphenyl)methylene]hydrazide) and Ln(acac)3·H2O (Hacac = acetylacetone) in different reaction solutions, namely [Ln4(CO3)(L)4(acac)2(H2O)4]·2CH3CN (Ln = Gd(1), Dy(2)) and [Ln4(CO3)(L)4(acac)2(CH3OH)2(H2O)2]·CH3OH·H2O (Ln = Gd(3), Dy(4)). Interestingly, complexes 1–4 are nearly structurally identical except for the coordinated solvent molecules. All of them consist of a Ln4 cluster in the center, in which four Ln(III) ions are bridged by two μ2-O atoms from the in situ formed CO32− through spontaneous fixation of atmospheric CO2. Magnetic investigation suggests that complexes 1 and 3 display magnetic refrigeration behaviors with maximum values of magnetic entropy changes of 31.23 J kg−1 K−1 and 27.06 J kg−1 K−1, respectively. Additionally, the energy barriers of the magnetization reversal were significantly improved from 2.73 K for 2 to 23.79 K for 4 by deliberately using methanol to replace the coordinated H2O molecule. It should be noted that the divergence in structures and magnetic properties could be mainly ascribed to the different solvent effects in the synthesis process.


 Please wait while we load your content...
Please wait while we load your content...