Highly efficient Pb(ii) and Cu(ii) removal using hollow Fe3O4@PDA nanoparticles with excellent application capability and reusability†
Abstract
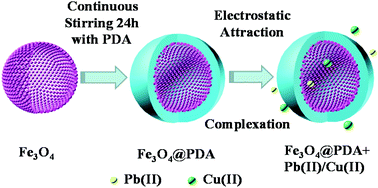
Potentially toxic metals in sewage and industrial effluents pose a serious threat to human health and the environment. Herein, core–shell hollow magnetic polydopamine nanoparticles (denoted as Fe3O4@PDA) were fabricated via a simple one-pot synthesis method and applied for the elimination of Pb(II) and Cu(II) from wastewater. The versatile polydopamine (PDA) layer with abundant functional groups (amine, imine and catechol groups) provided favourable sites to bind metal ions. The experimental results showed that the adsorption processes were significantly affected by the pH values of the suspension. The ionic strength-independent adsorption processes indicated the existence of inner-sphere surface complexes of Pb(II) and Cu(II) on Fe3O4@PDA. The results showed that Fe3O4@PDA exhibited fast removal kinetics for Pb(II) and Cu(II), which achieved equilibrium within 3 h. The adsorption processes were well fitted by the pseudo-second-order kinetic model and Langmuir isotherm model. Furthermore, the removal capacities of Fe3O4@PDA were calculated to be 57.25 mg g−1 for Pb(II) and 86.35 mg g−1 for Cu(II), which were much higher than those of pure Fe3O4 and other materials. Most importantly, the adsorption efficiencies of Pb(II) and Cu(II) on Fe3O4@PDA were still ∼71% and ∼70% after five cycles, respectively. In summary, the hollow Fe3O4@PDA nanoparticles can be used as outstanding materials for the elimination of Pb(II) and Cu(II), which is beneficial to reduce the toxic effects of heavy metal ion contaminated water.


 Please wait while we load your content...
Please wait while we load your content...