Spent alkaline battery-derived manganese oxides as efficient oxygen electrocatalysts for Zn–air batteries†
Abstract
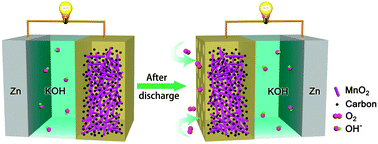
Manganese oxides were derived from spent commercial AA-type alkaline Zn–MnO2 batteries (ZMBs) via a facile route and applied as effective oxygen electrocatalysts for Zn–air batteries (ZABs). After leaching in dilute acid and annealing in air at mild temperature, the cathode dismantled from the battery discharged to 0.8 V is converted from γ-MnO2 into ε-MnO2, which features abundant defects, a porous structure and a high surface area and shows high activity in catalyzing the oxygen reduction/evolution reaction (ORR/OER). Remarkably, the recycled manganese oxide from one ZMB is estimated to sustain 1000 coin-type ZABs, which could potentially deliver a total capacity of 675 A h and an energy of 810 W h. Additionally, a rechargeable ZAB assembled with the recycled ε-MnO2 exhibits a considerable charge–discharge voltage separation of 0.65 V at 2 mA cm−2. Furthermore, we show that the hydrothermally prepared α-MnO2 serves initially as a cathode in a ZMB and directly functions as an efficient air electrode in a ZAB at its discharged states. This study offers a unique way to synthesize active manganese oxide electrocatalysts and a promising strategy to recycle spent batteries.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...