Simulation of osmotic energy conversion in nanoporous materials: a concise single-pore model†
Abstract
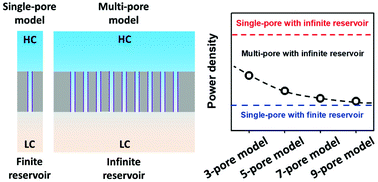
Salinity difference in ionic solutions is considered as a potential candidate for clean energy. Nowadays, nanofluidic reverse electrodialysis systems have received renewed attention for harnessing salinity gradient power. Towards practical applications, great efforts have been made in the fabrication of membrane-scale nanoporous materials. From a theoretical point of view, however, state-of-the-art simulation methods for multi-pore nanofluidic systems consume huge amounts of computational resources that frequently preclude simulation on lab-used computers. Here, we present a concise single-pore model to simulate the osmotic energy conversion in nanoporous materials. By regulating the geometric size of the solution reservoir, we show that the single-pore model is sufficiently accurate to simulate diffusive ion transport in multi-pore nanofluidic systems. More importantly, it largely reduces the computational scale by more than one order of magnitude. A benefit of this feature is that the model can incorporate more physical processes, such as the motion of fluid and heat conduction, which greatly expands the scope of the simulation method for understanding charge and mass transport behavior through nanoporous materials.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...