Towards oxalate-bridged iron(ii), cobalt(ii), nickel(ii) and zinc(ii) complexes through oxotris(oxalato)niobate(v): an open air non-oxidizing synthetic route†
Abstract
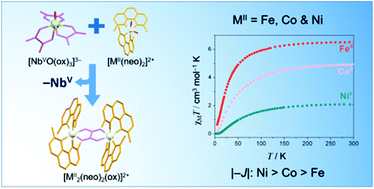
Four compounds with the formula [M2(dmphen)4(μ-C2O4)](ClO4)2·2dmso [M = Fe (1), Co (2) and Zn (4); dmphen = 2,9-dimethyl-1,10-phenanthroline] and [Ni2(dmphen)4(μ-C2O4)]3[NbO(C2O4)3]2·16H2O (3) have been synthesized using the tris(oxalato)oxoniobate(V) complex anion as the oxalate source, and their structures have been determined by single crystal X-ray diffraction. X-ray quality crystals of highly insoluble oxalate-bridged species were obtained by taking advantage of the slow release of oxalate by the tris(oxalato)oxoniobate(V) complex anion. The structures of 1–4 all contain oxalate-bridged dimetal(II) units with didentate dmphen molecules acting as end-cap ligands; electroneutrality is achieved by perchlorate (1–4) and oxotris(oxalato)niobate(V) (3) anions. Each divalent metal ion in 1–4 is tris-chelated in a six-coordinate distorted octahedral environment. The niobium(V) ion in 3 is seven-coordinate in a distorted pentagonal bipyramidal geometry built from one oxo group and six oxygen atoms from three didentate oxalate ligands. The values of the metal–metal separation across the bis-chelating oxalate are 5.626(1) (1), 5.575(1) (2), 5.434(1) to 5.447(1) (3) and 5.603(1) Å (4). The cryomagnetic measurements in the temperature range of 2.0 to 300 K for compounds 1–3 show the occurrence of antiferromagnetic interactions between the divalent metal ions across the oxalate bridge. The nature and amplitude of these magnetic interactions are rationalized by simple symmetry considerations and compared with those previously reported for related oxalate-bridged systems.


 Please wait while we load your content...
Please wait while we load your content...