The electrochemical storage mechanism in oxy-hydroxyfluorinated anatase for sodium-ion batteries†
Abstract
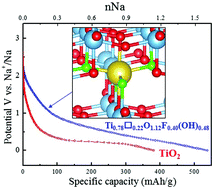
Replacing lithium ions with sodium ions as the charge carriers in rechargeable batteries can induce noticeable differences in the electrochemical storage mechanisms of electrode materials. Many material parameters, such as particle size, morphology, and the presence of defects, are known to further affect the storage mechanism. Here, we report an investigation of how the introduction of titanium vacancies into anatase TiO2 affects the sodium storage mechanism. From pair distribution function analysis, we observe that sodium ions are inserted into titanium vacancies at the early stage of the discharge process. This is supported by density functional theory calculations, which predict that sodium insertion is more favourable at vacancies than at interstitial sites. Our calculations also show that the intercalation voltage is sensitive to the anion coordination environment of the vacancy. Sodiation to higher concentrations induces a phase transition toward a disordered rhombohedral structure, similar to that observed in defect-free TiO2. Finally, we find that the X-ray diffraction pattern of the rhombohedral phase drastically changes depending on the composition and degree of disorder, providing further comprehension on the sodium storage mechanism of anatase.


 Please wait while we load your content...
Please wait while we load your content...