Preparation of sulfide solid electrolytes in the Li2S–P2S5 system by a liquid phase process†
Abstract
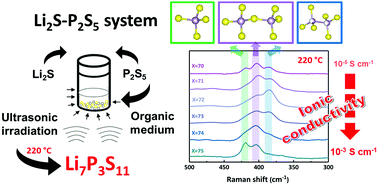
Sulfide solid electrolytes in the Li2S–P2S5 system were synthesized by a liquid phase process under ultrasonic irradiation, and heat treatments at low temperatures. Crystal phase, structure, morphology and ionic conductivity of the sulfide electrolytes were examined after solvent removal at 180 °C and two different heat treatment temperatures, 220 °C and 250 °C. The study revealed that the ionic conductivities of the xLi2S·(100 − x)P2S5 sulfide electrolytes, in compositions with 70 ≤ x ≤ 75 mol%, are largely influenced by the local structure. The heat treatment at 220 °C was found to be an adequate temperature to promote crystallization of the high ionic conductive Li7P3S11 phase. Higher temperatures for heat treatment such as 250 °C lead to the formation of P2S64− (hypo-thiodiphosphate) units in the local structure of the sulfide electrolytes leading to a reduction of ionic conductivity. The formation and distribution of PS43− (ortho-thiophosphate), P2S74− (pyro-thiophosphate) and P2S64− units in the local structure were found to be key factors in achieving higher ionic conductivity (up to 10−3–10−4 S cm−1 at room temperature).


 Please wait while we load your content...
Please wait while we load your content...