Hydrothermal shape controllable synthesis of La0.5Sr0.5MnO3 crystals and facet effect on electron transfer of oxygen reduction†
Abstract
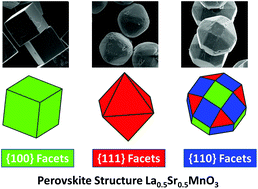
Controllable growth of perovskite structure oxide crystals with well-defined facets is challenging, especially in the mixed-valence state manganites with both rare-earth and alkaline-earth cations in their A-site. In this paper, we systematically studied the effects of the synthetic conditions of mineralizer concentration, reaction temperature and facet directing agent on the crystal shape formation of La0.5Sr0.5MnO3. The shapes of single crystal La0.5Sr0.5MnO3 have been controllably grown as cube, octahedron, rhombicuboctahedron and sphere by a high concentration KOH mineralized mild hydrothermal method with urea (CO(NH2)2) as crystal facet directing agent. The crystal growth mechanism for different facets has been analyzed according to Bravais−Friedel−Donnay−Harker theory. The as-grown facets for the different shapes of crystals indicate that the surfaces of La0.5Sr0.5MnO3 are composed of {100}, {110} and {111} facets. Electrochemical cyclic and linear sweeping voltammetry measurement by rotating ring-disk electrode of these crystals indicates a facet-dependent process for oxygen reduction catalysis. This work provides a useful strategy for growing high index crystal facets of complex oxides and materials for crystal-facet dependent physico-chemical applications.


 Please wait while we load your content...
Please wait while we load your content...