A novel route to synthesize carbon spheres and carbon nanotubes from carbon dioxide in a molten carbonate electrolyzer
Abstract
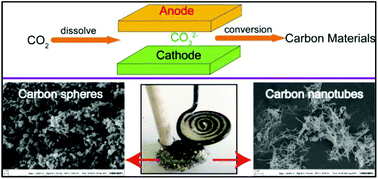
The process of molten salt CO2 capture and electrochemical conversion provides us with a new way to close the present carbon cycle and mitigate global climate change by transforming the greenhouse gas CO2 into carbonaceous fuels or chemicals. In this paper, carbon spheres and carbon nanotubes that can be used as a societal resource to serve mankind are synthesized from CO2 in diverse electrolyte composites with inexpensive metallic electrodes. Carbon products, subsequent to electrolysis, are characterized by EDS, SEM, TEM, Raman, TGA, FTIR, BET and XRD to reveal the elemental composition and morphological and structural features. The results demonstrate that Li–Ca–Na and Li–Ca–K carbonate electrolytes favor carbon sphere formation rather than carbon nanotube formation, and in particular, K2CO3 shows enhanced interference with carbon nanotube growth. In contrast, Li–Ca–Ba and Li–Ba carbonate composites present an increase in the carbon nanotube fraction. Additionally, CNTs generated from Li–K, Li–Ba and Li–Ca–Ba present a different diameter. In this way, the CO2-derived carbon products of carbon spheres and carbon nanotubes could be alternatively synthesized through the appropriate regulation of the electrolyte composition.


 Please wait while we load your content...
Please wait while we load your content...