Sustainable synthesis and precise characterisation of bio-based star polycaprolactone synthesised with a metal catalyst and with lipase†
Abstract
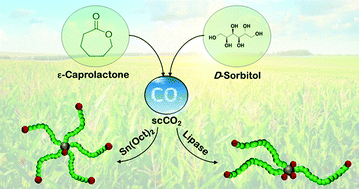
Bio-based building blocks and sustainable synthesis pathways were used to synthesise star-shaped polymers composed of a D-sorbitol core and polycaprolactone arms by ring opening polymerisation (ROP). The use of volatile organic solvents was avoided and less energy intense reaction conditions were achieved by performing the ROP in the bulk or in a green solvent, supercritical CO2 (scCO2). Two catalysts were tested: conventional tin(II) 2-ethylhexanoate (Sn(Oct)2) which is a Food and Drug Administration (FDA) approved metal catalyst and an enzyme, Novozym 435 (Lipase B from Candida Antarctica immobilised on a solid support). The influence of the reaction medium and of the nature of the catalyst on the molecular weight, the dispersity and the architecture of the PCL stars was investigated. The star polymers were characterised by 1H and 31P nuclear magnetic resonance (1H and 31P NMR) spectroscopy, size exclusion chromatography – multi-angle light scattering (SEC-MALS) and matrix-assisted laser desorption and ionisation-time of flight (MALDI-TOF) mass spectrometry. The use of scCO2 enabled the reduction of the reaction temperature of Sn(Oct)2 catalysed star D-sorbitol-polycaprolactone polymerisations from 140 to 95 °C. In addition, star polymers were successfully synthesised by enzyme catalysis in the bulk or in scCO2 at 60 °C; lower temperatures that could provide significant energy savings on a commercial scale. The catalyst was shown to have a pronounced influence on the architecture of the PCL stars. Regular star polymers were obtained in the presence of Sn(Oct)2 whereas Novozym 435 gave access to miktoarm-type star PCL. Finally, the influence of the number and length of the arms on the thermal properties of the star polymers was investigated by differential scanning calorimetry (DSC).


 Please wait while we load your content...
Please wait while we load your content...