Study of the mechanoluminescence and ‘aggregation-induced emission enhancement’ properties of a new conjugated oligomer containing tetraphenylethylene in the backbone: application in the selective and sensitive detection of explosive†
Abstract
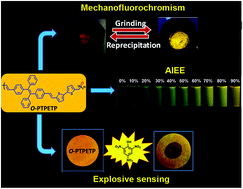
The development of new conjugated polymers with ‘aggregation-induced emission (AIE)’ characteristics has been of great interest in recent years to avoid the ‘aggregation-caused quenching (ACQ)’ effect and due to their potential applications in PLEDs, chemosensors and bio-imaging. In this work, we have developed a D–π-A-based AIE conjugated oligomer (oTPETP) derived from tetraphenylethylene (TPE) building blocks via a Wittig reaction. The electron-rich bithiophene moiety as a donor and TPE as an acceptor were alternately linked via π conjugation. This alternate D–A structure through π conjugation in the oligomer chain enables intramolecular charge transfer or twisted intramolecular charge transfer (ICT/TICT) interactions between the D and A moieties. Computational studies of a single repeating unit of the oligomer support that the transition is ICT/TICT. The structures of the synthesized compounds and oligomer were characterized by FT-IR, NMR and GPC techniques with good results. The obtained oligomer was readily soluble in common organic solvents such as chloroform, dichloromethane and tetrahydrofuran etc. The oligomer shows weak emission in solution after dissolving it in a solvent and a strong emission is observed in the aggregated state as a result of its ‘Aggregation-Induced Emission Enhancement (AIEE)’ characteristics. This is due to the restricted intramolecular rotation (RIR). The oTPETP oligomer exhibits an abnormal blue-shifted mechanoluminescent effect in contrast to the TPE derivatives, which show red-shifted emission. The causes of this transition in the oligomer have been investigated using PXRD and DSC techniques. In addition, oTPETP has been successfully tested in the sensing of picric acid through photoluminescence (PL) quenching.


 Please wait while we load your content...
Please wait while we load your content...