Ligand-free iron-based electrochemically mediated atom transfer radical polymerization of methyl methacrylate†
Abstract
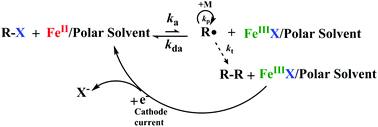
Iron-based simplified electrochemically mediated atom transfer radical polymerization (seATRP) was successfully developed by using a sacrificial counter electrode and discarding organic nitrogen or phosphorus ligands. The catalytic activity of FeCl3·6H2O could be modulated just by different kinds of polar solvents (NMP, DMI, DMF, MeCN, and PEG200). With all of these solvents, polymerization of methyl methacrylate was effective and well-controlled with the agreement of theoretical molecular weight with experimental values, linear first-order kinetics, and narrow molecular weight distributions. This system is particularly intriguing because (i) expensive and toxic ligands are not required, (ii) the whole polymerization process could be operated in air, (iii) the activity is high even when catalyst loading is reduced down to 70 ppm and with low solvent content, and (iv) the reaction can be conducted in biocompatible and less expensive PEG200. Also, the effects of the magnitude of the applied potential, the species of catalysts, the monomer/solvent ratio and the initiators were also studied in detail.


 Please wait while we load your content...
Please wait while we load your content...