Schiff base and reductive amination reactions of α-amino acids: a facile route toward N-alkylated amino acids and peptoid synthesis†
Abstract
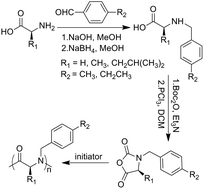
Polypeptoids are a promising class of peptidomimetic polymers for applications in biotechnology, but the polymers prepared by solution polymerization have limited side-chain functionalities due to synthetic difficulty. Synthetic versatility still remains challenging. Herein, we demonstrate a facile approach to prepare N-substituted amino acids and peptoid polymers via Schiff base and reductive amination reactions from readily available natural α-amino acids. These N-substituted amino acids can be easily converted into the corresponding N-substituted N-carboxy anhydrides (NNCAs), which subsequently undergo ring-opening polymerization (ROP) to prepare polypeptoids. Two NNCA monomers, i.e., N-(4-methylphenyl)methyl glycine (NBnMe-G) and N-(4-ethylphenyl)methyl glycine (NBnEt-G) NCAs, can be polymerized to give peptoid oligomers, P(NBnMe-G)10 and P(NBnEt-G)10. Also, the corresponding diblock copolypeptoids, mPEG45-b-P(NBnMe-G)12 and mPEG45-b-P(NBnEt-G)10, were successfully synthesized via methoxypolyethylene glycol amine (mPEG-NH2) initiated ROP. The thermal properties of these oligopeptoids and diblock copolymers were investigated. The synthetic strategy represents a new methodology to directly install N-substituents onto α-amino acids towards the functional polypeptoids.


 Please wait while we load your content...
Please wait while we load your content...