Quantitative studies on the p-substituent effect of the phenolic component on the polymerization of benzoxazines
Abstract
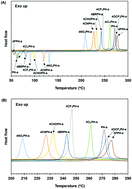
The pure monofunctional benzoxazines substituted by either electron donating or withdrawing groups are synthesized to verify the electronic effect on the polymerization behaviors without any complicated factors of the impurities. The analytical data of each compound are collected using 1H-NMR, 13C-NMR, and differential scanning calorimetry (DSC). In order to quantify the electronic effect on the polymerization behavior, the Hammett substituent constant is utilized and plotted against resonances of 1H-NMR, 13C-NMR spectra and DSC exotherm maximum temperature. The use of the Hammett substituent constant is reexamined by calculating the natural charge on the phenolic moiety via ab initio calculation using the Gaussian program and correlated with the polymerization exotherm temperature. The activation energies obtained using the Kissinger and Ozawa methods are related to the electronic effect of the substituents on the phenolic part.


 Please wait while we load your content...
Please wait while we load your content...