Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization†
Abstract
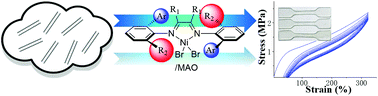
Due to a unique chain walking process, α-diimine nickel catalysts produce variously branched polyolefins using only ethylene as the feedstock. Ligand structures and polymerization conditions can exert influences over polymer microstructures, which in turn determine the mechanical properties of the polymeric products. In this contribution, a series of unsymmetrical α-diimine ligands bearing both aryl and dibenzhydryl moieties are synthesized and characterized. The corresponding nickel complexes are highly active in ethylene polymerization when activated using methylaluminoxane (up to 8.8 × 106 g of PE (mol of Ni)−1 h−1). These nickel complexes are stable over a wide temperature range (30–90 °C) and are capable of generating polyethylenes with a very high molecular weight (Mn up to 1.8 × 106 g mol−1) and variously branched structures (48 to 79 branches per 1000 carbon atoms). These metal complexes also show appreciable activities in the polymerizations of propylene, 1-hexene, 1-octene and 1-decene. Furthermore, they are demonstrated to mediate efficient propylene copolymerizations with methyl 10-undecenoate and 10-undecen-1-ol, affording polar functionalized polyolefins. With the appropriate choice of nickel complexes and polymerization conditions, polyethylene products with great tensile and elastic properties can be obtained.


 Please wait while we load your content...
Please wait while we load your content...