Balancing steric and electronic effects of bidentate, mixed P,N ligands to control Kumada catalyst transfer polycondensation of a sterically hindered thiophene†
Abstract
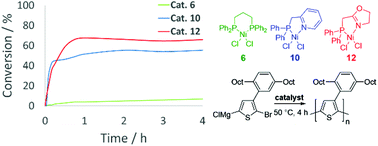
The polymerization of sterically hindered thiophenes via Kumada catalyst transfer polycondensation (KCTP) has been shown to be challenging to control using catalysts with bidentate diphosphine ligands but promising with mixed P,N ligands. Herein we present a broad range of NiII and PdII complexes bearing either diphosphines or hybrid P,N ligands for catalyzing the KCTP of 2-bromo-3-(2,5-dioctylphenyl)-5-iodothiophene. Among these, catalytic activity and control over the reaction was optimized by a subtle balance of sterics and electronics of the mixed P,N ligand. The best performance was obtained with an oxazoline-based P,N ligand. Density functional theory calculations indicate that the association energies between Ni0 and the polythiophene backbone are higher for hybrid P,N ligands compared to diphosphines and highest for the oxazoline-based P,N ligand. This explains the reduced tendency for dissociation, resulting in decreased chain termination and higher conversion. This study broadens the range of available catalysts for KCTP useful for the polymerization of sterically hindered thiophene monomers.


 Please wait while we load your content...
Please wait while we load your content...