Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization†
Abstract
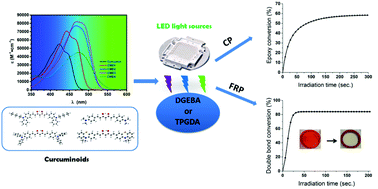
Curcumin is attractive in photopolymerization especially in cationic photopolymerization, because of its broad absorption and nontoxic properties. Curcuminoid (CMD) is a linear diarylheptanoid and can be considered as a derivative of curcumin. Based on the framework of curcumin, four CMDs containing carbazole/triphenylamine/dimethylaniline/phenothiazine as electron donors and β-diketone as electron acceptors are prepared using a facile synthesis method. The absorption maxima of the prepared CMDs all showed significant red-shifts compared with that of curcumin. The CMDs can act as high performance photosensitizers of iodonium salt (ONI) to start the ring-opening cationic photopolymerization of epoxides and the free radical photopolymerization of acrylates upon blue-green light exposure using light-emitting diodes. Excellent polymerization initiating efficiencies are found, and high final function conversions are obtained. The initiating abilities of the CMDs/ONI can be enhanced in the presence of N-vinyl carbazole or triphenylphosphine. More remarkably, the CMDs/ONI exhibit excellent photobleaching properties, and can be used in deep free radical photopolymerization.


 Please wait while we load your content...
Please wait while we load your content...