Polymers from sugars and CS2: synthesis and ring-opening polymerisation of sulfur-containing monomers derived from 2-deoxy-d-ribose and d-xylose†
Abstract
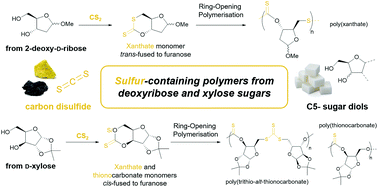
Thionocarbonate (–O–CS–O–) and xanthate (–S–CS–O–) cyclic monomers were synthesised by cyclothiocarbonation of 2-deoxy-D-ribose- and D-xylose-derived diols with carbon disulfide, then polymerised using organocatalytic ring-opening methods. Regular polymer linkages were obtained, with the sugar backbone influencing the regioselectivity of monomer opening. Thermal analysis revealed lower glass transition temperatures compared to carbonate analogues and a low onset of thermal degradation.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...