Direct access to biocompatible nitroxide containing polymers†
Abstract
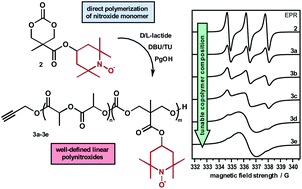
We introduce the preparation and ring-opening polymerization of a nitroxide containing carbonate monomer. Using 1,8-diazabicyclo undec-7-ene (DBU) and a thiourea as the catalyst system, we demonstrate that the free radical moiety of the monomer has no influence on the ring-opening copolymerization with D/L-lactide in a wide range of compositions between 10% and 80% of the nitroxide monomer, which can be readily controlled by the comonomer composition in the polymerization mixture. The copolymers exhibit narrow and monomodal mass distributions with number average molar masses between 6000 g·mol−1 to 7000 g·mol−1 and low dispersities below 1.1. The free radical functionality is retained during the polymerization as demonstrated by electron spin resonance (EPR) spectroscopy. High-resolution mass spectrometry coupled to size exclusion chromatography allowed an in-depth analysis of the copolymers showing well-defined linear polymer chains with excellent endgroup fidelity. Furthermore, the molar mass of the polymers was controlled by the monomer to initiator ratio which led to copolymers of up to 19 500 g·mol−1.


 Please wait while we load your content...
Please wait while we load your content...