Direct synthesis of poly(benzoxazine imide) from an ortho-benzoxazine: its thermal conversion to highly cross-linked polybenzoxazole and blending with poly(4-vinylphenol)†
Abstract
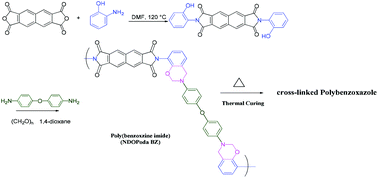
This paper describes a facile one-pot synthesis of a poly(benzoxazine imide), NDOPoda Bz, through the reaction of a difunctional naphthalene dianhydride ortho-phenol (ND-ortho-phenol), paraformaldehyde, and 4,4′-oxydianiline in 1,4-dioxane, without the need for either the preparation of an amino-functionalized benzoxazine or subsequent thermal treatment. This new poly(benzoxazine imide) underwent cross-linking polymerization to form a highly cross-linked poly(benzoxazine imide), which, with additional thermal treatment, was converted to highly cross-linked polybenzoxazoles. A model monomer (NDOPa Bz) was also synthesized based on the reaction of ND-ortho-phenol with paraformaldehyde and aniline. Moreover, we investigated the blending of NDOPoda Bz with a poly(4-vinylphenol) (PVPh) homopolymer at various weight ratios to form miscible blend systems. Fourier transform infrared spectroscopy, differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA) revealed the thermal stability and thermal curing behavior of these blends, which were miscible because of strong hydrogen bonds between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of PNDOPoda BZ and the OH groups of PVPh. The DSC and TGA data suggested that these hydrogen bonds enhanced the glass transition temperatures, thermal stability, and char yields of the blends. In addition, this approach decreased the temperature for ring opening of the benzoxazine, accelerated the rate of benzoxazole ring formation of NDOPda Bz at a lower temperature, and improved the thermal stability of the formed polybenzoxazoles.
O groups of PNDOPoda BZ and the OH groups of PVPh. The DSC and TGA data suggested that these hydrogen bonds enhanced the glass transition temperatures, thermal stability, and char yields of the blends. In addition, this approach decreased the temperature for ring opening of the benzoxazine, accelerated the rate of benzoxazole ring formation of NDOPda Bz at a lower temperature, and improved the thermal stability of the formed polybenzoxazoles.


 Please wait while we load your content...
Please wait while we load your content...