Poly(ester)–poly(silyl methacrylate) copolymers: synthesis and hydrolytic degradation kinetics†
Abstract
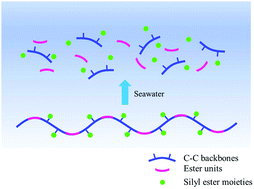
Copolymerization via a radical ring opening polymerization of 2-methylene-1,3-dioxepane (MDO) with various silyl methacrylates including bis(trimethylsiloxy)methylsilyl methacrylate (MATM2), triisopropylsilyl methacrylate (TIPSiMA) and tributylsilyl methacrylate (TBSiMA) has been reported. Kinetics of polymerization show that the monomers react in a near-linear manner during the early hours and the consumption of silyl monomers is faster than MDO. The silyl ester side groups in the copolymers are prone to hydrolysis on contact with artificial seawater (ASW), leading to a decrease of the water contact angle of the copolymer film with immersion time. The nature of the silyl ester groups was shown to be able to tune the hydrolysis rate of the copolymers and the hydrophilicity of polymer films. The hydrolysis rates of MATM2 and TBSiMA are faster than the TIPSiMA one. The water uptake test shows that copolymers containing MATM2 and TBSiMA have higher water absorption than the copolymer based on TIPSiMA. MDO copolymers with MATM2 and TBSiMA show a significant decline in molar mass by 90% and 86% after immersion in ASW at room temperature for 7 weeks, mainly due to the cleavage of main-chain ester bonds. Their degradation is accelerated at 60 °C; meanwhile the TIPSiMA-based polyester does not show notable degradation at both temperatures, indicating that the hydrolytic degradation rate of the polymer backbone is tuned by the amount of hydrolyzed ester side groups.


 Please wait while we load your content...
Please wait while we load your content...