Alternating ring-opening copolymerization of phthalic anhydride with epoxides catalysed by salophen chromium(iii) complexes. An effect of substituents in salophen ligands†
Abstract
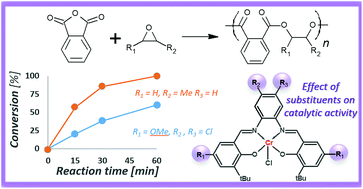
The effect of a ligand structure on the catalytic activity of salophen chromium(III) complexes in the ring-opening copolymerization of phthalic anhydride with a series of epoxides was studied. The catalytic activity of ten differently substituted complexes was explored in the copolymerization reactions conducted in toluene solutions using equimolar mixtures of monomers. The experiments were performed both with and without DMAP addition as a co-catalyst. The presence of F or Me substituents in the moieties of 1,2-phenylenediamines increased the activity of the complexes. An increase in the complex activity was also found with decreasing the donor character of substituents in salicylaldehyde moieties (in the order OMe < tBu < H). When the molar ratio of [PA] : [epoxide] : [Cr] : [DMAP] = 250 : 250 : 1 : 1 was applied, the most active complexes allowed the copolymerization of phthalic anhydride with differently substituted epoxides (cyclohexene oxide, 4-vinylcyclohexene oxide, styrene oxide, phenyl glycidyl ether, propylene oxide, butylene oxide, epichlorohydrin) quantitatively within 60 minutes at 110 °C. The resulting polyesters were characterized by Mn up to 19 kg mol−1 and narrow dispersity, and they did not contain polyether units.


 Please wait while we load your content...
Please wait while we load your content...