Synthesis and properties of poly(trifluoroethylene) via a persistent radical mediated polymerization of trifluoroethylene†
Abstract
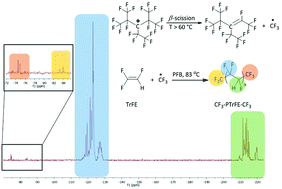
The conventional radical homopolymerization of TrFE initiated by a ˙CF3 radical (generated in situ from perfluoro-3-ethyl-2,4-dimethyl-3-pentyl persistent radical, PPFR) is presented. Reactions were carried out at an increasing PPFR concentration (initial [TrFE]0 : [PPFR]0 molar ratios varying from 2.5 to 20.0 mol%) to influence the molar masses (Mn, ranging between 7700 and 38 100 g mol−1). PTrFE homopolymer yields were high (76–87%). The microstructures of such synthesized PTrFEs were characterized by 1H and 19F NMR spectroscopy. The generated PTrFE chains were exclusively terminated by CF3 end-groups, as revealed by MALDI-TOF spectroscopy and NMR analysis, indicating that the ˙CF3 radicals initiated the polymerization of TrFE. The addition of the ˙CF3 radicals onto TrFE was predominantly regioselective (66%) leading mainly to a CF3–CHF–CF2–PTrFE homopolymer. Furthermore, the CF3 end group could be used as an efficient label to determine the PTrFE molar masses by 19F NMR spectroscopy that also enabled us to assess the chain defects (14%). The thermal properties of these CF3–PTrFE–CF3 polymers revealed (i) a high thermal stability (their degradation under an oxidative atmosphere started from 362 °C and ranged even up to 428 °C for higher molar masses of PTrFEs) and (ii) a semi-crystalline behavior with a crystallinity rate averaging 36%.


 Please wait while we load your content...
Please wait while we load your content...