Synthesis of indocyanine green functionalized comblike poly(aspartic acid) derivatives for enhanced cancer cell ablation by targeting the endoplasmic reticulum†
Abstract
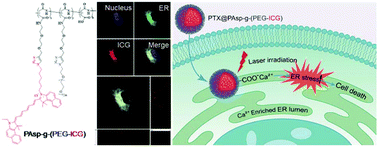
Undesired gene expression can lead to severe diseases such as cancers. Oncogenic proteins or survival factors encoded by oncogenes can sustain cancer cell survival and proliferation. Considering that proteins are manufactured and transported by the endoplasmic reticulum (ER), the destroying of oncogenic proteins within the ER could cut-off the process of undesired gene expression. In this study, a new type of ER targeting strategy was developed based on the coordination interaction of the Ca(II) ion rich ER lumen with the carboxyl group of poly(aspartic acid) (PAsp). A comblike polymer PAsp-g-(PEG-ICG) was rationally designed and successfully prepared by grafting azido-modified polyethylene glycol (PEG) and the azido-modified photosensitizer indocyanine green (ICG) onto alkynyl-modified PAsp through the copper(I)-catalyzed alkyne–azide cycloaddition (CuAAC). Here, ICG not only shows its importance as an imaging agent that reveals the distribution of PAsp-g-(PEG-ICG) micelles both at tissue and subcellular levels, but also acts as a protein destructive agent to generate reactive oxygen species (ROS) resulting in protein denaturation. Moreover, chemotherapeutic drugs, such as paclitaxel, can be easily encapsulated into PAsp-g-(PEG-ICG) (PTX@PAsp-g-(PEG-ICG)) in up to 28% drug loading capacity with excellent stability. The experimental results proved that the as-prepared PTX@PAsp-g-(PEG-ICG) micelles exhibited selective accumulation in the ER lumen of cancer cells. Thus, a 10-fold enhancement of ROS was obtained by incubating cancer cells with PAsp-g-(PEG-ICG) micelles at a PTX concentration of 1.0 μg ml−1 under laser irradiation (0.2 W cm−2, 785 nm, 30 s) for 24 h, leading to disruption of proteins in the ER that caused ER stress-induced cancer cell apoptosis up to 76%. In a cytotoxicity test, U-87 MG glioma cells incubated with PTX@PAsp-g-(PEG-ICG) at the PTX concentration of 2.5 μg ml−1 were reduced to nearly 0% of cell viability upon 24 h laser irradiation (2 W cm−2, 785 nm, 30 s), in comparison with free PTX that showed 60% cell viability under the same conditions, indicating that photodynamic therapy (PDT) remarkably enhanced chemotherapeutic effects. With the good combination of PDT and chemotherapy, the PTX@PAsp-g-(PEG-ICG) micelles offer unprecedented advantages by effectively targeting the ER and displayed a prolonged retention time in the tumors of U-87 MG bearing nude mice, providing great promise for manipulating biomaterial design to achieve the intended ER targeted delivery for non-invasive cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...