RAFT polymerization of a RGD peptide-based methacrylamide monomer for cell adhesion†
Abstract
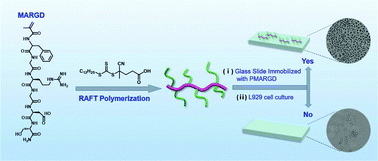
Synthetic peptides containing the arginine–glycine–aspartate (RGD) motif have been used extensively as inhibitors of integrin–ligand interactions in studies of cell adhesion and cell target moiety. MARGD (sequences: Phe-Gly-Arg-Gly-Asp-Ser), a methacrylamide monomer containing a pending RGD peptide, was synthesized and obtained in high purity (≥90%) via an automated peptide synthesizer followed by a simple precipitation in diethyl ether. MARGD can be polymerised by reversible addition–fragmentation chain transfer (RAFT) polymerization to afford well-defined polymers containing a RGD peptide group with controlled molecular weight, low dispersity (Đ < 1.25), and a precise chain end structure. In this study, linear pseudo-first-order kinetics and number average molecular weight dependence on conversion were observed during the RAFT polymerization. Diblock peptide-polymer conjugates were prepared using PMARGD as a macro-chain transfer agent or using MARGD as a monomer, and the resulting diblock conjugates all showed low dispersities. The cytotoxicity study using mouse fibroblast cells (L929) revealed that the bioconjugates are non-toxic up to high concentration. Furthermore, enhanced cell adhesion was observed when the bioconjugates immobilized on the glass slide surfaces. This study provides a novel and efficient strategy to access well-defined peptide-polymer conjugates with diversity for both peptides and polymers.


 Please wait while we load your content...
Please wait while we load your content...