Temperature responsive poly(phosphonate) copolymers: from single chains to macroscopic coacervates†
Abstract
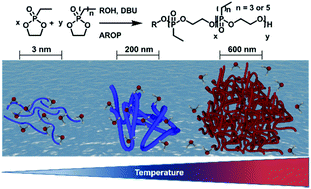
We present the first series of random poly(ethylene alkyl phosphonate) copolymers with either high solubility in water or a finely tunable hydrophilic-to-hydrophobic phase transition upon heating (“LCST”). Polymerization via the organocatalytic anionic ring-opening polymerization provided high control over molecular weight (up to 23 000 g mol−1) and copolymer composition, and resulted in narrow molecular weight distribution (Đ < 1.3). Polymers of molecular weights up to 23 000 g mol−1 were obtained. The phase separation temperature was precisely adjusted in a range from 55 °C to 6 °C in water, depending on the copolymer composition. The phase transition mechanism was thoroughly investigated at different length scales via electron paramagnetic resonance spectroscopy (EPR), dynamic light scattering (DLS), UV-Vis spectroscopy and confocal laser scanning microscopy (cLSM), proving the step-wise formation of aggregates close to the cloud point temperature up to macroscopic coacervates.


 Please wait while we load your content...
Please wait while we load your content...