Fluorescence behaviour of 2-, 3- and 4-amino-1,8-naphthalimides: effects of the substitution positions of the amino functionality on the photophysical properties†
Abstract
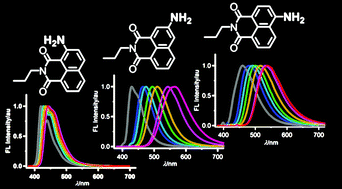
The absorption and fluorescence spectra of a series of 1,8-naphthalimide derivatives incorporating the amino functionality at the 2-, 3- and 4-positions of the naphthalene ring (2APNI, 3APNI and 4APNI, respectively) were systematically investigated in various solvents and in the solid state. The fluorescence spectra of 2APNI were insensitive to solvent polarity and intermolecular hydrogen-bonding even in a protic medium such as methanol. Thus, 2APNI displayed blue fluorescence with a moderate fluorescence quantum yield (λFmax = 420–445 nm, ΦF 0.2–0.3) in the solvents investigated. In contrast, the fluorescence spectra of 3APNI and 4APNI were strongly solvent dependent showing positive solvatofluorochromism with large Stokes shifts. Upon increasing the solvent polarity, the fluorescence colours changed from blue in hexane (λFmax = 429 nm) to orange-yellow in methanol (λFmax = 564 nm) for 3APNI, and from blue in hexane (λFmax = 460 nm) to yellow in methanol (λFmax = 538 nm) for 4APNI. The fluorescence quantum yields of 3APNI and 4APNI decreased with increasing solvent polarity. In the solid state, APNIs displayed red-shifted fluorescence emission compared to that in solution (λFmax = 541 nm for 2APNI, 575 nm for 3APNI, and 561 nm for 4APNI) and the fluorescence quantum yields in the solid state were lower than those in solution.


 Please wait while we load your content...
Please wait while we load your content...