Photoisomerization of di-nuclear rhenium(i) bpe-based compounds†
Abstract
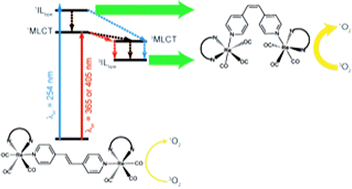
Di-nuclear [{(NN)(CO)3Re}2(trans-bpe)](PF6)2 complexes, NN = 4,7-diphenyl-1,10-phenanthroline (ph2phen) or 4,7-dichloro-1,10-phenanthroline (Cl2phen) and trans-bpe = trans-1,2-bis(4-pyridyl)ethylene, were synthesized and characterized by 1H NMR and UV-visible spectroscopy. Irradiation of acetonitrile solutions led to 1H NMR and UV-visible spectral changes ascribed to trans-to-cis photoisomerization processes showing that the presence of another Re(I)-moiety does not preclude the photoisomerization process. Quantum yields for the di-nuclear isomerization of [{(ph2phen)(CO)3Re}2(trans-bpe)]2+ were higher than those for the corresponding mono-nuclear compound process. Additionally, the higher quantum yield obtained with 254 nm irradiation for [{(Cl2phen)(CO)3Re}2(trans-bpe)]2+ showed, for the first time, that the singlet pathway could also be accessed in addition to the usually observed triplet one. The luminescence in fluid solution observed for the [{(NN)(CO)3Re}2(cis-bpe)]2+ complexes is able to efficiently photosensitize the generation of singlet O2.


 Please wait while we load your content...
Please wait while we load your content...