Chromo-luminescent selective detection of fluoride ions by a copper(ii) bis(terpyridine) complex solution via a displacement approach†
Abstract
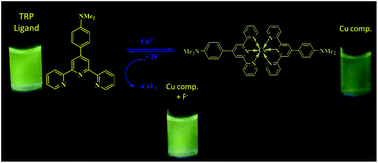
Herein, for the first time, we have reported a copper(II) bis(terpyridine) complex solution for instant ‘naked eye’ chromo-luminescent selective detection of fluoride ions in an acetonitrile medium at micromolar concentration. The copper complex [Cu(II) (L)2] (NO3)2 [where L = 4′-(4-N,N′-dimethylaminophenyl)-2,2′:6′,2′′-terpyridine] was characterized by mass spectroscopy and the terpyridine ligand by 1H NMR spectroscopy. The complex solution selectively discriminates F− ions from other anions such as AcO−, Br−, Cl−, CN−, H2PO4−, HSO4−, and I− in acetonitrile media via exceptional optical changes. The optical changes were evaluated by UV-visible and fluorescence techniques. Studies on the binding characteristics of the copper complex solution with fluoride ions revealed a displacement of copper ions from the complex solution as CuF2 resulting in the significant optical changes. Furthermore, displacement of Cu(II) from the complex was established by means of mass spectroscopy in the presence of 20 equivalents of fluoride ions. The limit of detection (LOD) was found to be 5.07 μM which is within the permissible range of fluoride ions in drinking water set by the World Health Organization (WHO).


 Please wait while we load your content...
Please wait while we load your content...