A little key to oxalate formation in oil paints: protective patina or chemical reactor?†
Abstract
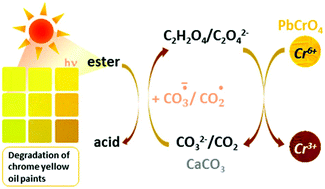
By means of synchrotron based techniques, we propose an integrated mechanism for the degradation of 19th century chrome yellow oil paints based on pigment reconstructions from historical recipes. We show that for certain paint formulations the darkening of these colours is triggered by the binder photodegradation which leads to the formation of calcium oxalate at the expense of the filler CaCO3, and the reduction of the chrome yellow pigment (Cr6+/Cr3+). Considering that calcium oxalate is formed as a thin superficial layer, that may prevent light absorption by the paint bulk, we discuss its role as protective patina.


 Please wait while we load your content...
Please wait while we load your content...