SuperQuat chiral auxiliaries: design, synthesis, and utility
Abstract
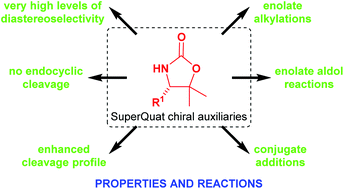
The SuperQuat (4-substituted 5,5-dimethyloxazolidine-2-one) family of chiral auxiliaries was first developed by us in the 1990s to address the shortcomings of the Evans (4-substituted oxazolidin-2-one) family of chiral auxiliaries. The incorporation of geminal dimethyl substitution at C(5) has two effects: (i) it induces a conformational bias on an adjacent, otherwise conformationally labile C(4)-substituent so that it projects towards the N-acyl fragment, thus offering superior diastereofacial selectivity in a range of transformations; and (ii) it hinders nucleophilic attack at the endocyclic carbonyl group, facilitating recovery and recyclability of the auxiliary, with enhanced cleavage properties. This review summarises the development and some of the most common uses of the SuperQuat family of chiral auxiliaries, particularly in the synthesis of natural products or other targets having bioloigcal interest. Where possible, comparisons with the performances of the corresponding Evans auxiliaries are presented.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...