B(C6F5)3-catalyzed Markovnikov addition of indoles to aryl alkynes: an approach toward bis(indolyl)alkanes†
Abstract
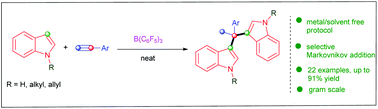
The first example of the metal- and solvent-free B(C6F5)3-catalyzed Markovnikov addition of indoles to aryl alkynes was disclosed. Both N–H and N-protected indoles were tolerated, leading to a wide spectrum of versatile bis(indolyl)alkanes in moderate to good yields with high regioselectivities.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...