Practical regio- and stereoselective azidation and amination of terminal alkenes†
Abstract
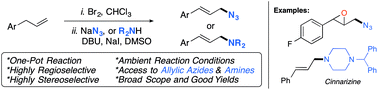
There is significant interest in developing more rapid and efficient production of nitrogen-containing allylic compounds, as widely used in various syntheses. This work reports a variety of allylic azides and allylic amines synthesized by an efficient, new one-pot protocol that employs readily available terminal alkenes as starting materials. This method is highly regio- and stereoselective, affording the linear (E)-isomer, under metal-free conditions. This process tolerates several functional groups including halogen-containing molecules; it is general for azides and amine nucleophiles; and, adducts were obtained in good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...