Asymmetric synthesis of highly functionalized spirothiazolidinone tetrahydroquinolines via a squaramide-catalyzed cascade reaction†
Abstract
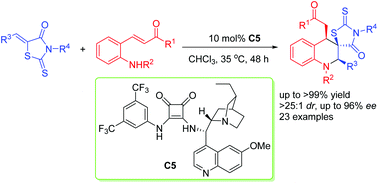
A bifunctional squaramide-catalyzed asymmetric cascade aza-Michael/Michael addition reaction for the synthesis of chiral spirothiazolidinone tetrahydroquinolines with three contiguous stereocenters has been developed. This cascade reaction proceeded well under mild conditions, and afforded the desired products in high to excellent yields (up to >99% yield) with excellent diastereoselectivities (>25 : 1 dr) and high enantioselectivities (up to 96% ee). More importantly, both the amplification and the derivation experiments do not affect the stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...