Synthesis of 4-trifluoromethyl 2-pyrones and pyridones through the Brønsted base-catalyzed Pechmann-type reaction with cyclic 1,3-diones†
Abstract
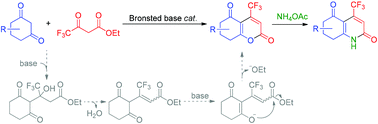
An efficient method for the synthesis of 4-trifluoromethyl 2-pyrones through the Brønsted base-catalyzed Pechmann-type reaction of cyclic 1,3-diones with ethyl 4,4,4-trifluoroacetoacetate is described. In the presence of 2-dimethylamino pyridine (2-DMAP) as a catalyst, the resulting 4-trifluoromethyl 2-pyrones are formed in good to excellent yields. Additionally, the reaction also provides 4-trifluoromethyl 2-pyridones by using the easily available NH4OAc as a source of NH3.
- This article is part of the themed collections: Synthetic methodology in OBC and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...