Organocatalytic asymmetric synthesis of highly substituted pyrrolidines bearing a stereogenic quaternary centre at the 3-position†
Abstract
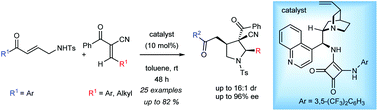
An organocatalytic asymmetric cascade reaction has been developed for the synthesis of highly substituted pyrrolidines having a stereogenic quaternary centre at the 3-position. N-Tosyl aminomethyl enone and trans-α-cyano-α,β-unsaturated ketone were utilized as the reaction partners in this method. Cinchonidine derived bifunctional amino-squaramide catalysts were the best to obtain the products in high enantio- and diastereoselectivities.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...